Contact-free ultrasound transfer of liquids down to 2.5 nanoliters – the Echo Liquid Handler
Revolutionary assay miniaturization lets you automate your research with high precision, speed and free of contamination

The most efficient and precise liquid handling technology for research
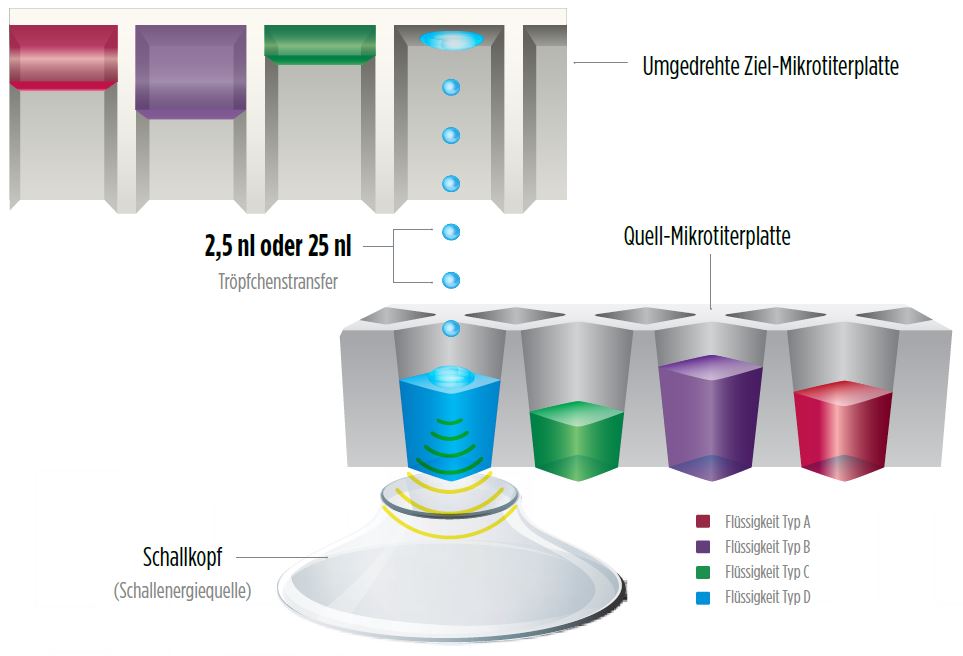
Echo ADE (acoustic droplet ejection) technology has revolutionized the liquid handling industry thanks to enabling the fast and highly precise transfer of liquids by acoustic energy without any physical contact. Acoustic pulses are used to dispense droplets of precise volume from the source plate to the target plate positioned above it.
Whether you are working in the development or management of drugs, in genomics, synthetic biology, proteomics, functional screening or other research areas, the acoustic droplet ejection technology will help you generate more reliable scientific results and save reagent costs.
The advantages of the Echo Acoustic Liquid Handler:
- Precise and flexible nanoliter transfers, from any position, to any position
- No risk of cross-contamination thanks to touchless sample handling
- Up to 100-fold cost savings¹ through assay miniaturization
- High throughput of up to 750,000 samples per day
- Integration into Access robot system possible
“As it sounds in, so it drops out …”
How Echo Acoustic Liquid Handling works
Revolutionize these applications with the Echo series:
- Drug discovery and development
- API management
- Genome research and sequencing
- Personalized medicine and functional screening
- Cancer research
- Synthetic biology
- Proteomics
Unique automation of your sample management workflows

Find all the applications, facts and figures in the Echo brochure

Life Sciences Division Headquarters
5350 Lakeview Parkway S Drive
Indianapolis, IN 46268
United States
Copyright/Trademark
Legal
Online Terms of Use
Privacy Statement
Do not sell personal data
Imprint
NOT ALL PRODUCTS ARE AVAILABLE IN ALL COUNTRIES.
PRODUCT AVAILABILITY AND REGULATORY STATUS DEPENDS ON COUNTRY REGISTRATION PER APPLICABLE REGULATIONS
The listed regulatory status for products correspond to one of the below:
IVD: In Vitro Diagnostic Products. These products are labeled “For In Vitro Diagnostic Use.”
ASR: Analyte Specific Reagents. These reagents are labeled “Analyte Specific Reagents. Analytical and performance characteristics are not established.”
CE: Products intended for in vitro diagnostic use and conforming to European Directive (98/79/EC). (Note: Devices may be CE marked to other directives than (98/79/EC)
RUO: Research Use Only. These products are labeled “For Research Use Only. Not for use in diagnostic procedures.”
LUO: Laboratory Use Only. These products are labeled “For Laboratory Use Only.”
No Regulatory Status: Non-Medical Device or non-regulated articles. Not for use in diagnostic or therapeutic procedures.
© 2000 – 2024 Beckman Coulter, Inc. All rights reserved.